Adhesion GPCRs
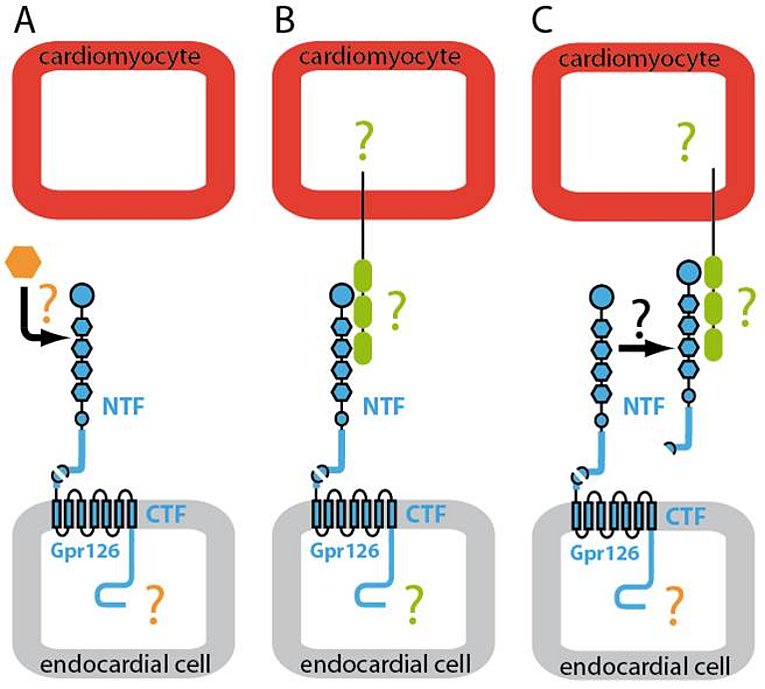
Gpr126 belongs to the large class of seven-transmembrane spanning (7TM) Adhesion-type G protein-coupled receptors (aGPCR). 7TM receptors have proven the linchpin for countless physiological functions and a treasure trove for modern pharmaceutical intervention. aGPCR appear in stark contrast to the rest of the 7TM receptor superfamily. Despite their abundance, remarkable size and molecular structure facilitating cell and matrix contacts in a variety of organ systems, aGPCR are by far the most poorly understood 7TM receptor class. They possess large N termini containing various functional domains. In addition, many of them are autoproteolytically cleaved at their GPS sites into an N-terminal fragment (NTF) and C-terminal fragment. However, neither the general biological and pharmacological properties of aGPCR are known, nor have they been utilized yet in biomedicine.
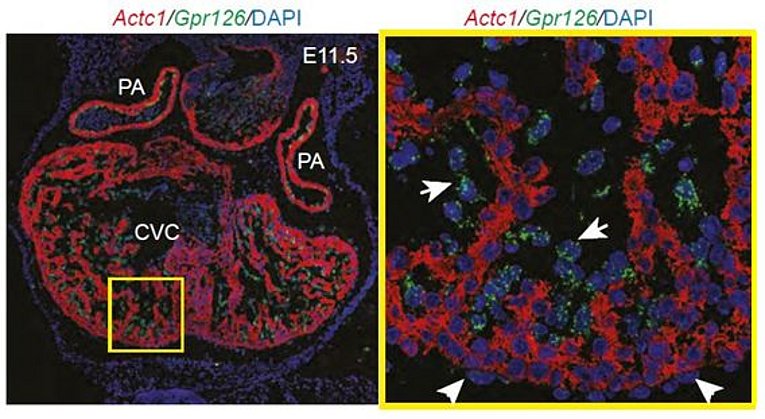
Previously, we have demonstrated that Gpr126 function is required for cardiac development. Gpr126 deletion or knockdown causes mitochondrial dysfunction and ventricular hypertrabeculation in mice and zebrafish. With the help of domain-specific morpholinos in zebrafish, rescue experiments in Gpr126-depleted morphants and an in situ protein binding assay using mGpr126-NTF on mouse tissue sections, we have demonstrated that the NTF fragment of Gpr126 is important for cardiac trabeculation. Our data suggested that mGpr126-NTF acts in a paracrine fashion. However, the molecular mechanism utilized by Gpr126 to regulate heart development remains elusive. Thus we currently work on the following questions:
- Identification of molecular changes underlying the heart phenotype of Gpr126-/- mice
- Characterize cell type-specific deletion of Gpr126 in conditional Gpr126-/- mice
- Establishment of in vitro assays to analyze Gpr126 function and signaling
- Structure-function analysis of Gpr126 in zebrafish
- Defining binding partners of NTF-Gpr126